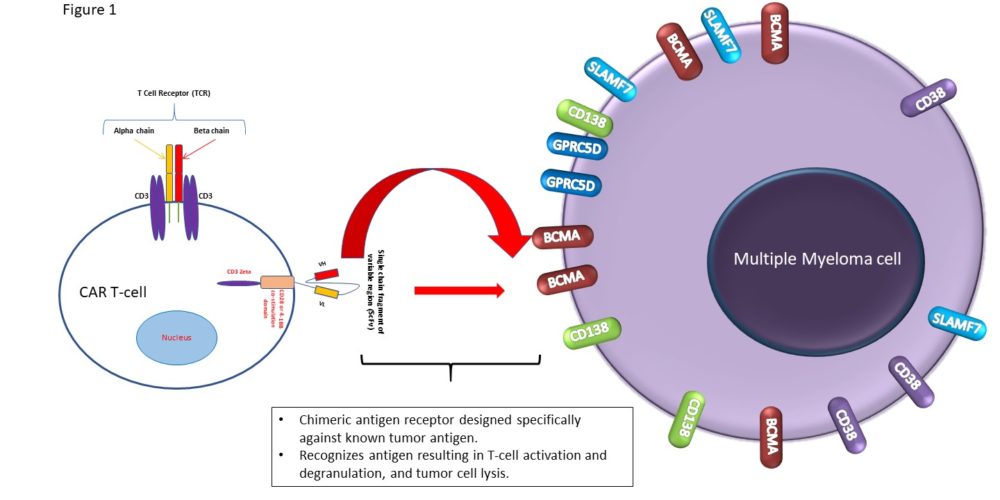
T-cell redirecting therapies for multiple myeloma treatment
Niels van de Donk, MD, PhD
VU University Medical Center
Triple-class refractory MM patients have a poor outcome and have an unmet medical need for new effective therapies. The LocoMMotion study provides real-world data on outcomes in triple-class exposed MM patients who receive standard-of-care therapies. The first analysis of this study showed that these triple-class exposed patients (74% triple-class refractory) had an overall response rate of only 20% with standard-of-care salvage therapies. This clearly indicates that we need new agents with novel mechanism of action for these patients. Promising in this respect are the different T-cell redirecting therapies that are currently under investigation in heavily pre-treated MM patients.
CAR T-cell therapy
Ide-cel is the first FDA-approved BCMA-specific CAR T-cell product. The approval was based on the KarMMa study in which patients with at least 3 prior regimens and previously exposed to an IMiD, PI, and CD38-targeting antibody received ide-cel after lymphodepletion with fludarabine/cyclophosphamide. In a subgroup analysis presented at ASCO/EHA it was demonstrated that the overall response rate was similar in patients with 3 prior lines of therapy or ≥4 prior lines of therapy (73% vs 73%). However, the complete response (CR) rate was higher in patients with 3 prior lines of therapy (53% vs 30%). This increased depth of remission did not translate into longer PFS (8.6 vs 8.9 months), probably due to a higher number of patients with high-risk cytogenetics and extramedullary disease in the subgroup of patients who received 3 prior treatment lines. Median OS for the whole cohort of patients was 24.8 months. The incidences of CRS and NT were similar in patients who received 3 or ≥4 prior lines of therapy.
There was also an update on the CARTITUDE-1 study at both conferences. In this study, mostly triple-class refractory patients, were treated with cilta-cel, which has a dual BCMA epitope-binding CAR construct, after fludarabine/cyclophosphamide lymphodepletion therapy. The overall response rate in 97 cilta-cel treated patients was 97.9% with at least CR in 80.4%. The median PFS for these patients was 22.8 months, and 18-month OS was 80.9%. Importantly, with longer follow-up there were no new safety signals observed and no new cases with neurotoxicity. Interestingly, at both meetings there was also a presentation about arm A from the CARTITUDE-2 study. Patients in this cohort were progressive after 1-3 prior lines of therapy and were lenalidomide-refractory. Nineteen of the 20 enrolled patients (95%) responded with CR in 75%. None of the patients experienced progression at a median follow-up of 5.8 months. CRS was mostly grades 1/2 and there were no events of movement and neurocognitive adverse events. This study forms the rationale for the ongoing phase 3 CARTITUDE-4 study: cilta-cel versus standard-of-care (DPd or PVd) in patients with 1-3 prior lines of therapy.
New mitigation strategies to prevent late neurotoxicty (enhanced bridging therapy to reduce tumor burden, early and aggressive treatment of CRS and ICANS, and handwriting assessments and extended monitoring) have shown to be effective, with no new cases of delayed neurotoxity with movement/neurocognitive symptoms after implementing this strategy.
Many more CAR T-cell studies are ongoing including trials evaluating CAR T-cells with a fully human antigen-binding domain, dual-targeting CAR T-cells, or allogeneic CAR T-cells. In addition, based on the promising results of both ide-cel and cilta-cel in heavily pretreated patients, several studies are ongoing and planned in patients with newly diagnosed disease or early relapse.
Bispecific antibodies
Several bispecific antibodies simultaneously targeting CD3 and BCMA are evaluated in heavily pretreated MM patients.
The BCMA-specific antibody teclisitamab has now been administered to a total of 157 patients: 84 received intravenous (IV) teclistamab and 73 subcutaneous (SC)administration. In the update presented at EHA/ASCO, data of the SC-treated patients was presented (40 treated at the RP2D of 1500 μg/kg SC). Teclistamab was well tolerated with no new safety signals. Hematologic toxicity mainly occurred during the first and second treatment cycle. The most common non-hematologic adverse event was CRS, which occurred a median of 1 day after the injection and was never grade 3 or higher. CRS also did not lead to treatment discontinuations. Although patients enrolled were heavily pretreated (approximately 80% triple-class refractory), the overall response rate of patients treated at the RP2D was 65% including ≥very good partial response (VGPR) in 58% and ≥CR in 40% of the patients. Responses are durable with at the RP2D 85% of the responders alive and continuing on treatment after a median follow-up of 7.1 months.
Elranatamab is another BCMA-specific bispecific antibody that was initially evaluated as an IV infusion, but in the most recent update also as a SC administration in 30 patients (86.7% triple-class refractory). At the RP2D (1000 μg/kg SC) the overall response rate was 83.3% (n=6 patients). Interestingly, responses were also reported in patients who were previously exposed to BCMA-targeted therapies. Most common adverse events were again hematologic toxicity and CRS.
GPRC5D is a protein that is highly expressed on the plasma cell and myeloma cell surface, and also on cells that produce keratin.
Talquetamab is the first-in-class GPRC5D-specific T-cell redirecting bispecific antibody. An update from the phase 1 MonumenTAL-1 study showed that at the time of data cut-off a total of 184 patients were enrolled (102 received IV dosing and 82 SC dosing). The RP2D has now also been determined for talquetamab and is defined as 405 μg/kg SC. Toxicities include again hematologic toxicity and CRS, but unique for GPRC5D targeting with talquetamab is skin toxicity and dysgeusia (probably related to GPRC5D expression in these tissues). However, these toxicities can be effectively managed with supportive care. Talquetamab was highly active in these heavily pretreated patients (approximately 80% triple-class refractory) with an ORR of 70% with ≥VGPR in 60% in the 30 patients treated at the RP2D.
FcRH5 is another myeloma-associated antigen, which forms the target for cevostamab. An EHA presentation showed that response to cevostamab was consistent regardless of previous therapies. FcRH5 expression was also not altered by the number of prior lines and or types of prior therapy.
These different bispecific antibodies are in further development as a monotherapy and some of these new agents also in combination with other anti-MM agents to further improve depth and duration of response. Studies are also planned in patients with newly diagnosed or disease or with early relapse.
Conclusion:
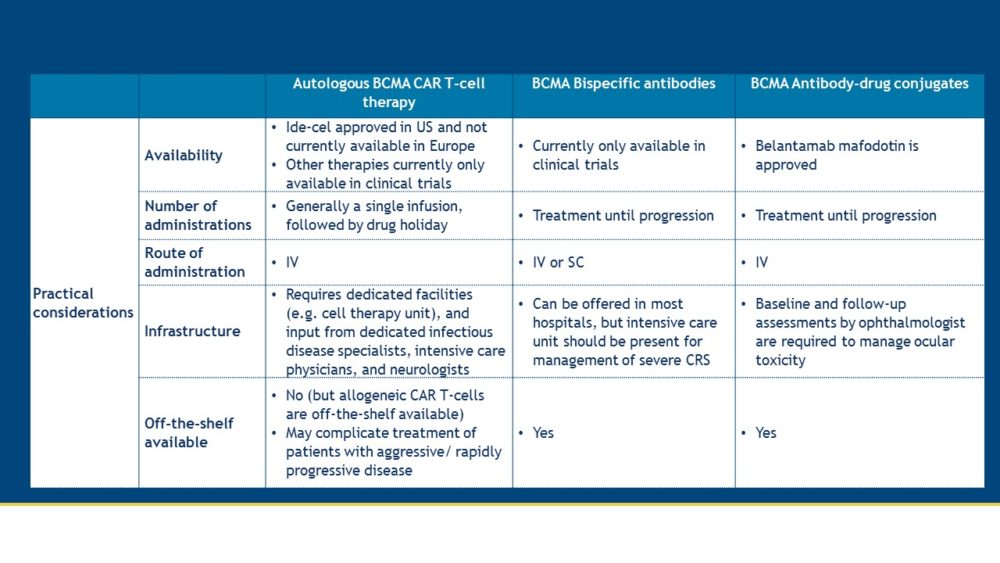
Both CAR T-cells and bispecific antibodies are promising new agents, which harness the power of T-cells to eliminate MM cells. The updates from EHA/ASCO 2021 demonstrate that these novel therapies for the treatment of MM patients show a beneficial balance between activity and toxicity. Various ongoing trials will inform us on several open questions, such as how to best sequence these new T-cell redirecting therapies and which patients will experience most benefit from these new drugs. Some differences in practical aspects between BCMA-specific CAR T-cells, bispecific antibodies, and antibody-drug conjugates are summarized in the table below.
Why Become a Member
The International Myeloma Society is a professional, scientific, and medical society established to bring together clinical and experimental scientists involved in the study of myeloma. The purpose of this society is to promote research, education, clinical studies (including diagnosis and treatment), workshops, conferences, and symposia on all aspects of multiple myeloma worldwide.
The IMS is a membership organization comprised of basic research scientists, and clinical investigators in the field along with physicians and other healthcare practitioners.