Targeting the Undruggable in Multiple Myeloma
Mariateresa Fulciniti, PhD
Dana-Farber Cancer Institute
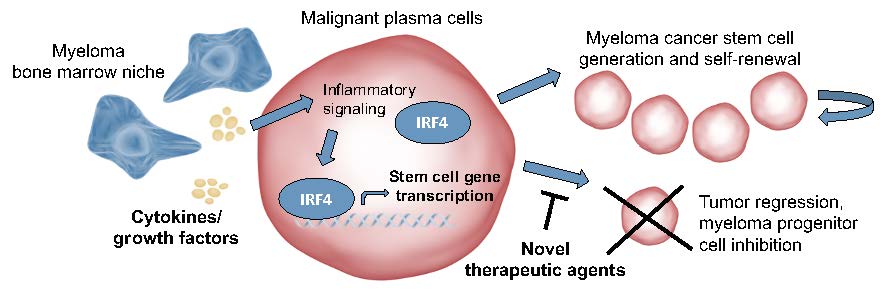
Cell identity is controlled by the action of a small number of master transcription factors (TFs) that recognize and bind specific sequences in the genome and thereby regulate specific gene expression programs. Tumor cells commonly have deregulated gene expression programs, yielding the concept of transcriptional addiction (https://pubmed.ncbi.nlm.nih.gov/28187285/). In MM, transcription factors establishing plasma cell identity such as IRF4 are aberrantly expressed in primary MM cells and represent a specific and potent dependency in MM cell line systems (https://pubmed.ncbi.nlm.nih.gov/18568025/). Moreover, the mechanism of action of standard-of-care IMiD therapies involves targeting the cereblon-Ikaros family zinc finger proteins-IRF4 (CRBN-IKZF-IRF4) protein degradation axis, confirming a key role for IRF4 in survival of myeloma cells (https://pubmed.ncbi.nlm.nih.gov/24292625/).
However, therapeutics capable of direct disruption of oncogenic TFs are yet lacking, contributing to the notion of undruggability of the transcriptional apparatus. In the study “Selective antisense oligonucleotide inhibition of human IRF4 prevents malignant myeloma regeneration via cell cycle disruption” published in Cell Stem Cell (https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(20)30601-9), the research team at the University of California San Diego School Medicine, in collaboration with scientists at Ionis Pharmaceuticals, utilize an antisense oligonucleotide (ASO)-based inhibitory platform to directly reduce stability of IRF4 transcripts in preclinical myeloma models. Selective inhibition of IRF4 with this investigational antisense oligonucleotide, ION251, lowers disease burden and increases survival of mice bearing human myeloma and reduces myeloma stem cell abundance. This agent also increased the sensitivity of myeloma cells treated with clinical drugs in the lab, suggesting that selective IRF4 inhibition may enhance the efficacy of other standard of care therapies.
“As a result of this comprehensive preclinical work, the team also helped identify a lead therapeutic agent that has recently advanced to a Phase 1 clinical trial for patients with relapsed or refractory multiple myeloma (https://clinicaltrials.gov/ct2/show/NCT04398485)” said lead author Leslie Crews. These proof-of-principle studies will hopefully enable rapid clinical development of antisense oligonucleotide-mediated IRF4 inhibition to prevent myeloma relapse driven by drug-resistant cancer stem cells.
According to Dr. Carl Novina of Dana-Farber Cancer Institute, this elegant study demonstrates the central role of IRF4 in myeloma progenitor cells. The heterogeneity of myeloma cells and the disseminated nature of this disease presents challenges to effective oligonucleotide-based therapies. However, identifying specific sub-populations of progenitor cells in a discrete location raises the intriguing possibility that antisense oligonucleotides targeting IRF4 may play critical roles in future myeloma therapies”.
Why Become a Member
The International Myeloma Society is a professional, scientific, and medical society established to bring together clinical and experimental scientists involved in the study of myeloma. The purpose of this society is to promote research, education, clinical studies (including diagnosis and treatment), workshops, conferences, and symposia on all aspects of multiple myeloma worldwide.
The IMS is a membership organization comprised of basic research scientists, and clinical investigators in the field along with physicians and other healthcare practitioners.